Biological Imaging Module (BIM)
What is The BIM?
The Biological Imaging Module provides UHCO researchers with wide range of instrumentation; transmission and scanning electron microscopy (including serial block-face sectioning), epifluorescence deconvolution, confocal (conventional and Airyscan), two-photon, light-sheet and atomic force microscopy, optical coherence tomographic imaging, in vivo retinal and corneal confocal imaging (HRT), image analysis software (Amira 6.2 and syGlass), and histological sample preparation. A highly skilled microscopist/histology technologist is available to coordinate use, assist with histology/microscopy and provide training.
Instrumentation

Transmission EM
FEI Tecnai Spirit BioTwin transmission electron microscope (TEM) equipped with a goniometer stage and two digital cameras (side and bottom mounts) for routine and tomographic imaging.

Scanning EM
Tescan Mira 3 scanning electron microscope (SEM) equipped with a Gatan 3View 2 ultramicrotome for serial block-face imaging.
Cornea Images
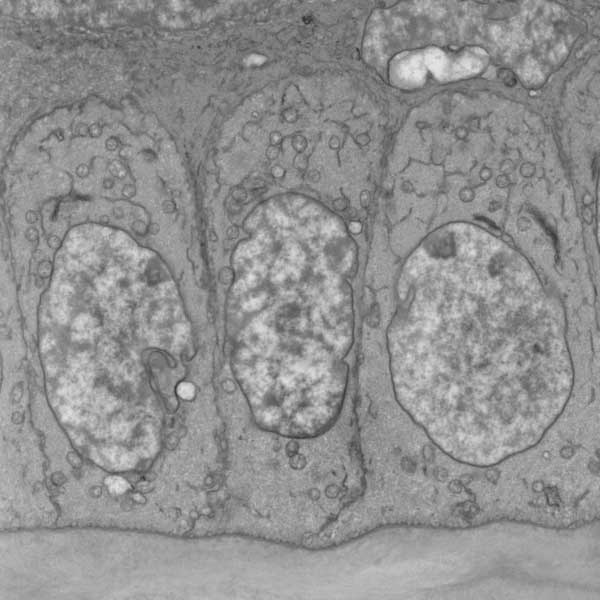
basal epithelial cells (left, SEM), Dr. Alan Burns
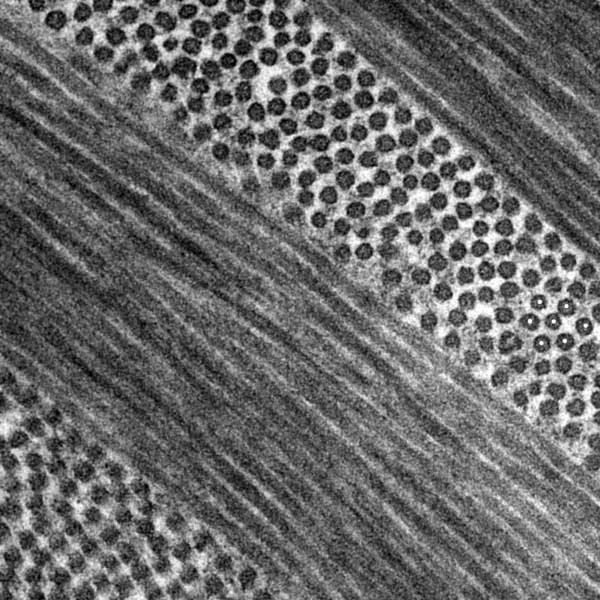
type I collagen fibrils organized into orthogonally arranged lamellae (middle, TEM) Dr. Alan Burns
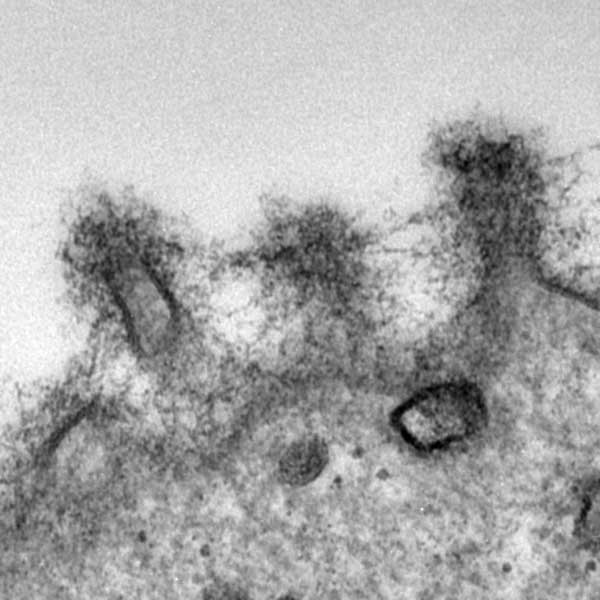
glycocalyx displayed on a superficial epithelial cell (right, TEM). Dr. Alan Burns

Airyscan Confocal LSM
Zeiss LSM 800 Airyscan confocal laser scanning microscopy (CLSM) system equipped for four channel fluorescence imaging plus transmitted light (DIC and bright field). Airyscan provides high-sensitive super-resolution microscopy.

Confocal LSM
Olympus FV3000 confocal laser scanning microscopy (CLSM) system equipped with six lasers and 4 detectors for multichannel spectral imaging. High speed imaging with resonant scanning is available.
Primate Retina Image
Image of primate retina captured by LSM800. Cone arrestin (green), vGlut1 (blue), Cx36 (red) and DAPI (white).
Dr. Nori Ishibashi
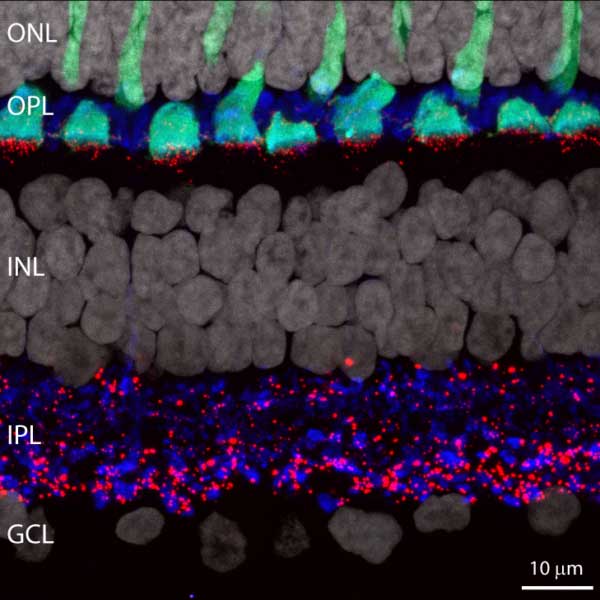

Section of Mouse Retina Image
Section of mouse retina labelled for all major neuronal classes: Photoreceptors (blue), Horizontal cells (pink), Bipolar cell (ON in green, OFF in red, RBC in orange), amacrine and ganglion cells (purple). Captured by FV3000.
Dr. Luca Della Santina

AFM
Bruker atomic force microscopy (AFM) system equipped with a Leica DMi8 inverted fluorescence microscope coupled with a BioScope Resolve atomic force microscope.

Widefield FM
DeltaVision Spectris Core deconvolution fluorescence microscopy (FM) system equipped with 4 channel imaging, transmitted light imaging, and sCMOS camera. Chamber system allows high-performance live-cell imaging.

Light-sheet FM
3i light-sheet fluorescence microscopy (LSFM) system with three sCMOS cameras. Dual inverted selective plane illumination (diSPIM) provides rapid 3D imaging with isotropic resolution.

Two-photon LSM
Scientifica two-photon excitation laser scanning microscopy (2PE-LSM) system with an upright microscope, a Spectra Physics InsightX laser, and patch clamp micromanipulators for recording from retinal whole mounts.

HRT
Heidelberg Retina Tomography (HRT) III enables in vivo confocal laser scanning microscopy (CLSM) for high-resolution images of the cornea and retina. HRT II is also available.

HRA+OCT
Heidelberg Spectralis optical coherence tomography (OCT) with angiography module for high-resolution imaging of the optic nerve head and retina.

SD-OCT
Bioptigen Envisu spectral domain optical coherence tomography (SD-OCT) system provides real-time high-resolution imaging of animals ranging from zebrafish to primate.

LSFG
Softcare laser speckle flowgraphy (LSFG)-LITE system provides real-time observation of blood flow changes in the fundus of rabbits, non-human primates, and pigs.
OCT / LSFG Captures
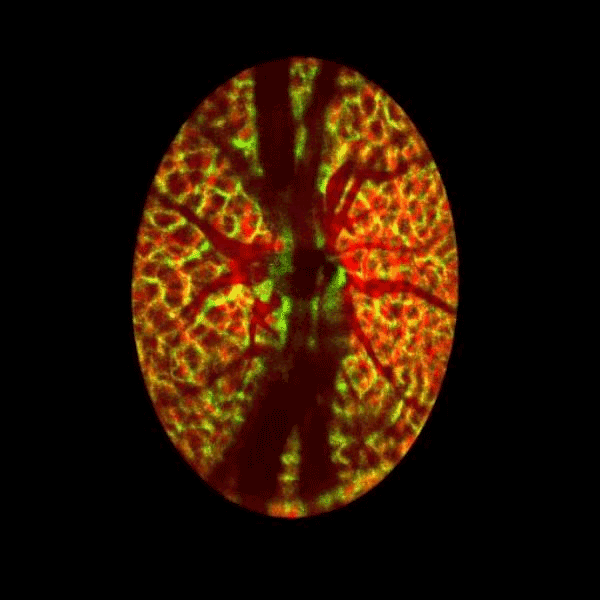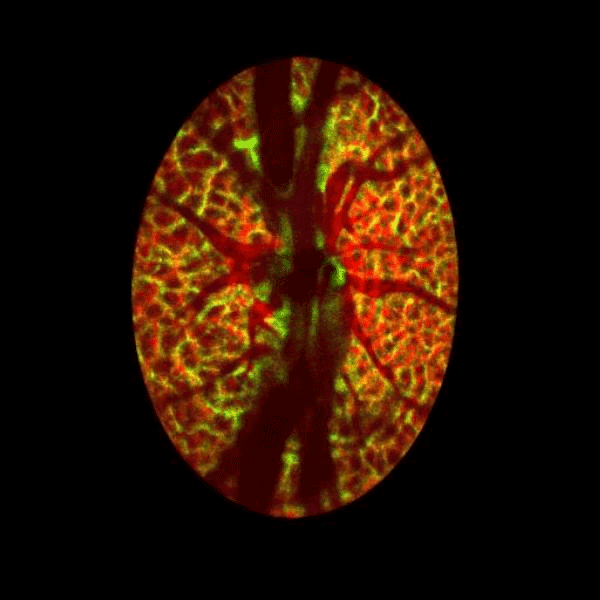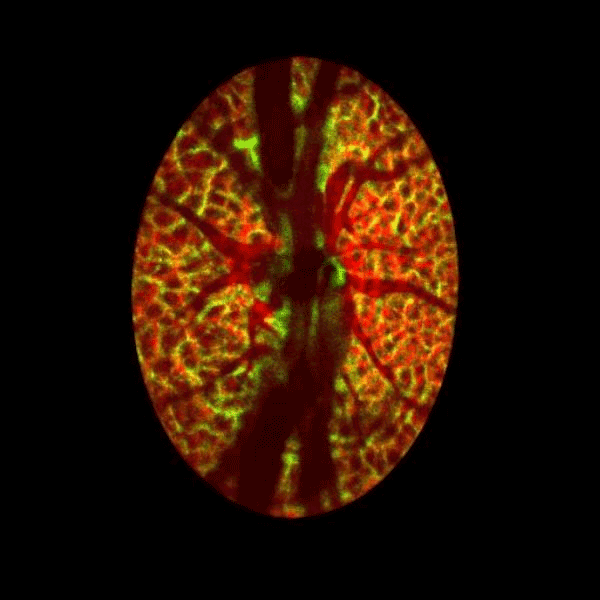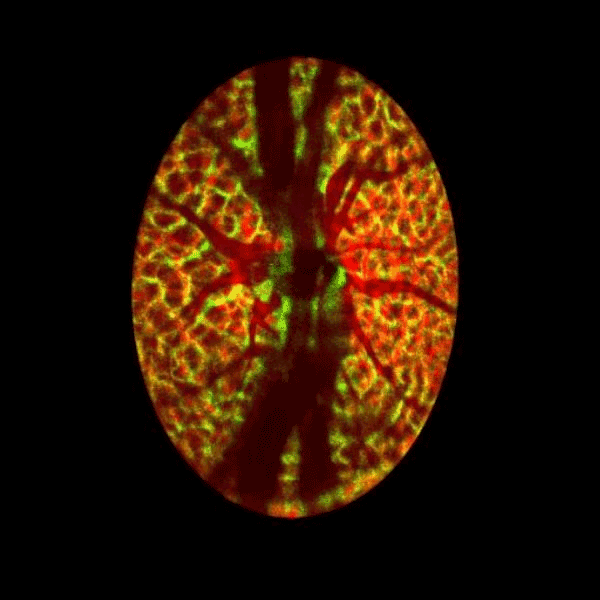
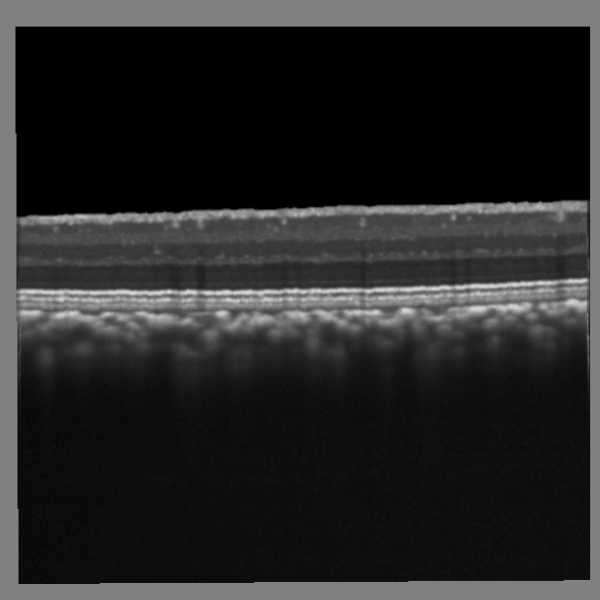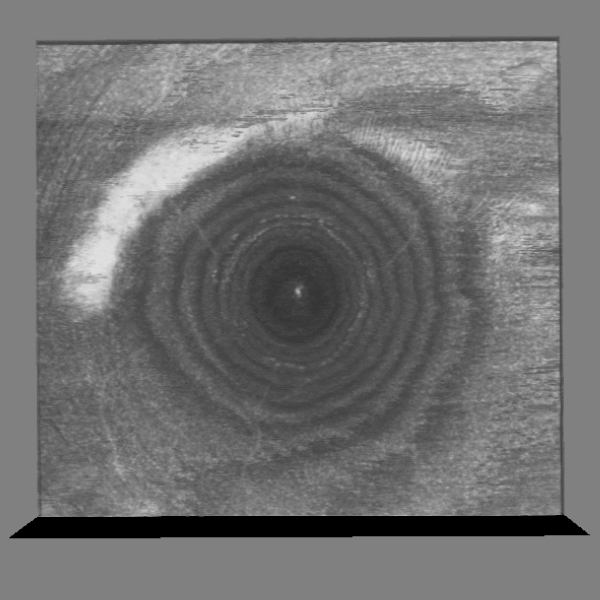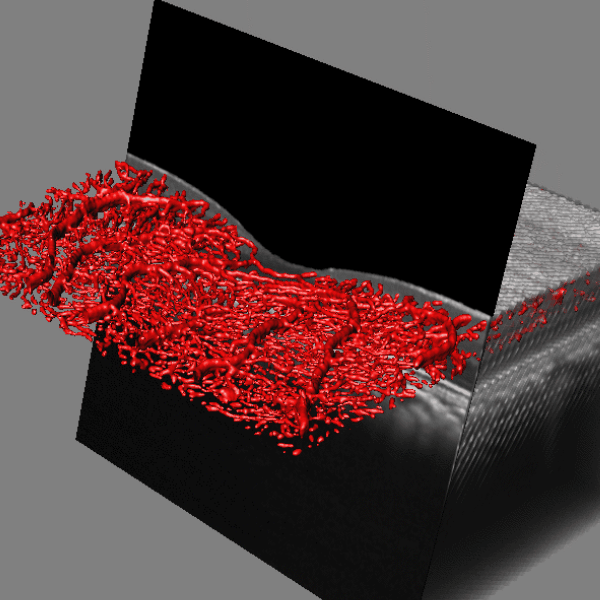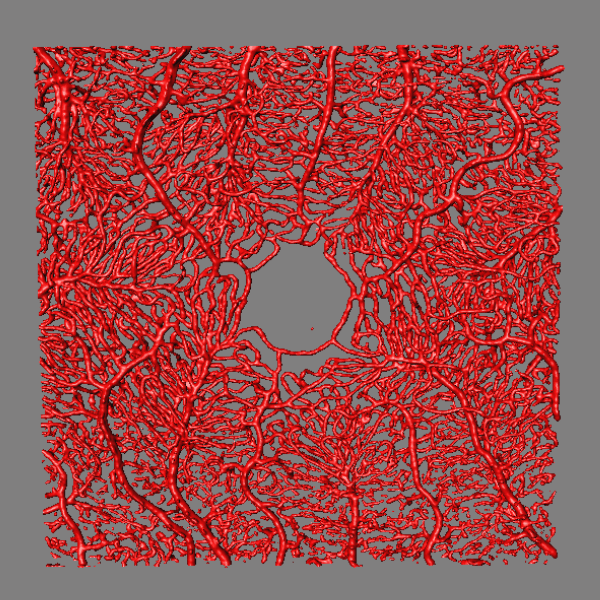
Lamina cribrosa structure (red) and vasculature (green) of a healthy non-human primate lamina
Structural and vascular imaging of the macula. Dr. Nimesh Patel
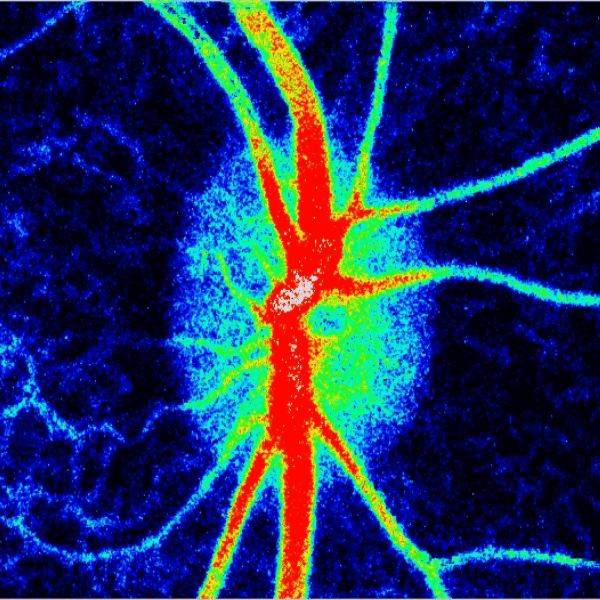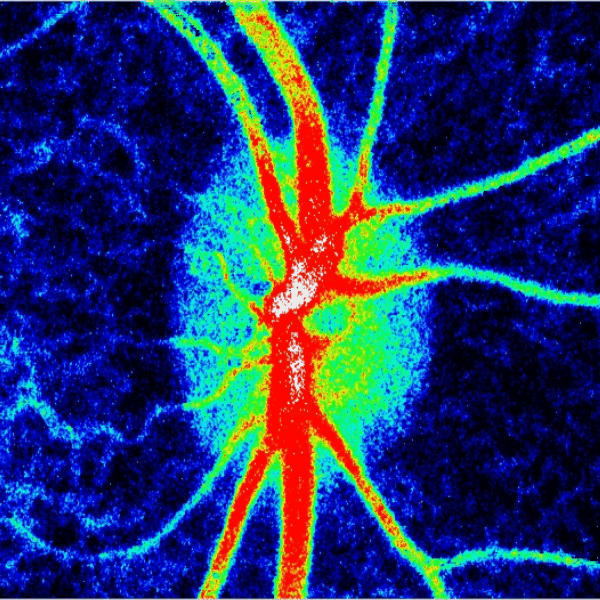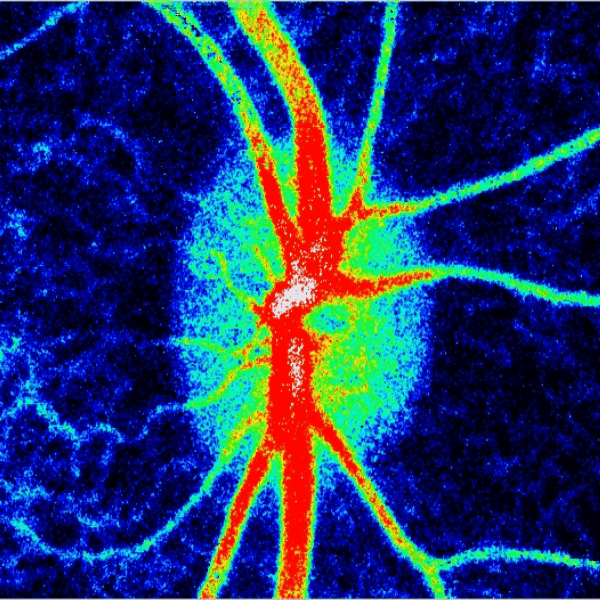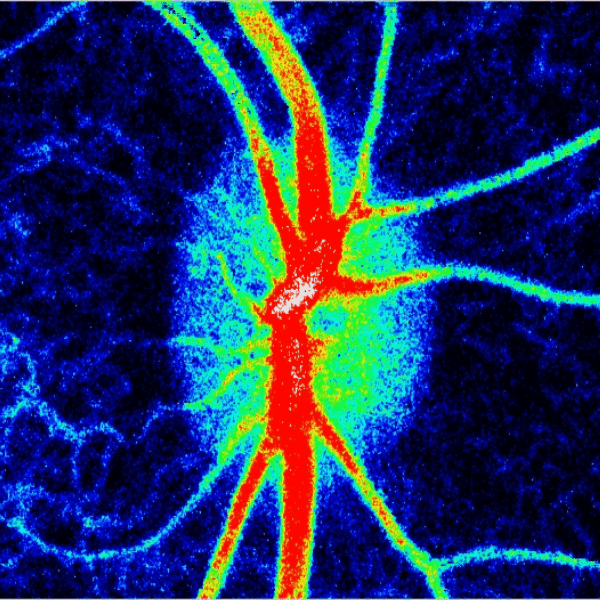
Blood flow of the optic nerve head Dr. Nimesh Patel
BIM - BY THE NUMBERS
2022
Financial Year
8
EM/LM/AFM - Microscopes
4
OCT/HRT/LSFG - Systems
26
Anually
55
In the last 5 years
3
Program Directors
Biological Imaging Module (BIM) is funded by NIH Center Core Grant (P30 EY007551). All users of BIM should cite the P30 grant in the Acknowledgement section of their publications. Please let us know when your paper is accepted.
Contact
Director

Nimesh Patel, O.D., Ph.D., FAAO
Office Number: JDA - 2156
Contact Dr Patel For advanced microscopy and image analysis collaborations
Co-Director

Alan R Burns, Ph.D.
Office Number: JDA - 2168
Contact Dr Burns for electron microscopy
Associate Director

Nori (Munenori) Ishibashi, Ph.D.
Office Number: JDA - 3374
Contact Dr. Ishibashi for light microscopy.

Location
University of Houston College of Optometry
J. Davis Armistead (JDA) Building
4401 Martin Luther King Boulevard
Houston, TX 77204-2020
